
 The Purdue College of Pharmacy is pleased to honor and recognize the outstanding research and scholarship generated by our faculty each month. This month we highlight Drs. Youchao Deng, Postdoc in Medicinal Chemistry and Molecular Pharmacology, and Rong Huang, Professor of Medicinal Chemistry and Molecular Pharmacology, for their recent publication, " Structure–Activity Relationship Studies of Venglustat on NTMT1 Inhibition", which can be read in the Journal of Medicinal Chemistry (January, 2023; DOI: 10.1021/acs.jmedchem.2c01854). Graduate students Guangping Dong, Ying Meng and Dr. Nicholas Noinaj (Professor of Biological Chemistry) also contributed to this research.
The Purdue College of Pharmacy is pleased to honor and recognize the outstanding research and scholarship generated by our faculty each month. This month we highlight Drs. Youchao Deng, Postdoc in Medicinal Chemistry and Molecular Pharmacology, and Rong Huang, Professor of Medicinal Chemistry and Molecular Pharmacology, for their recent publication, " Structure–Activity Relationship Studies of Venglustat on NTMT1 Inhibition", which can be read in the Journal of Medicinal Chemistry (January, 2023; DOI: 10.1021/acs.jmedchem.2c01854). Graduate students Guangping Dong, Ying Meng and Dr. Nicholas Noinaj (Professor of Biological Chemistry) also contributed to this research.
Protein methyltransferases are an important class of enzymes that regulate protein methylation levels, essential for numerous biological processes. Perturbing, the activities of protein methyltransferases, has been associated with cancers, metabolic, inflammatory, and neurodegenerative disorders. Thus, protein methyltransferases are regarded as novel therapeutic targets with multiple inhibitors in clinical trials. However, protein N-terminal methyltransferase 1 (NTMT1) is under-explored despite its implication in cancers and aging.
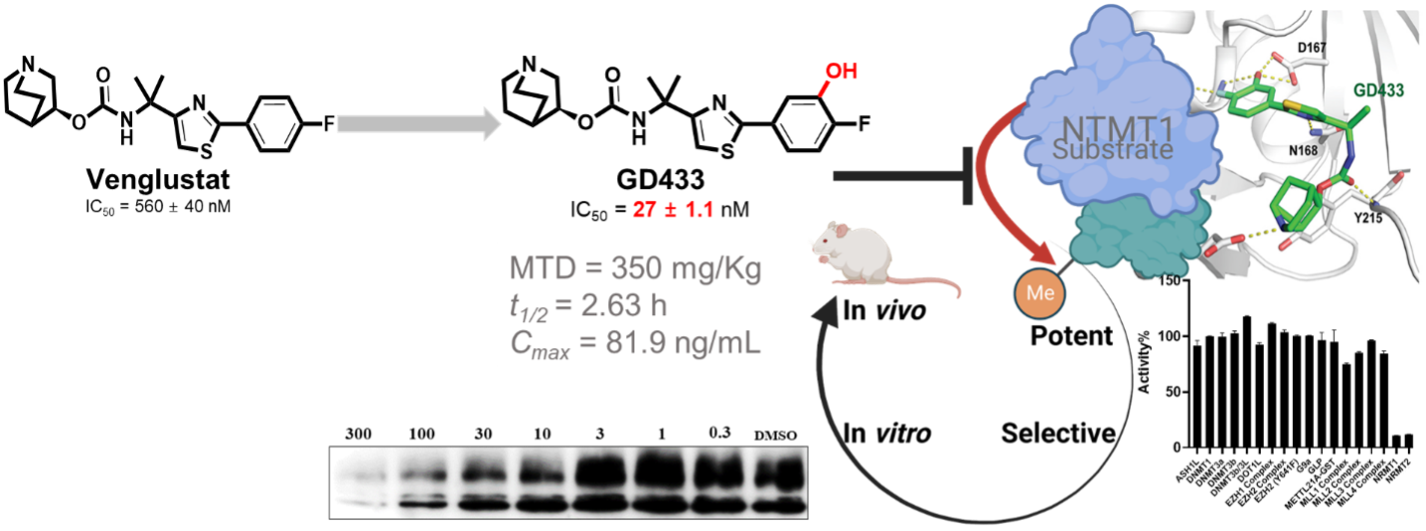 “There is no drug-like inhibitor for NTMT1, a novel but important methyltransferase for cell division and DNA repair,” Huang said. “Our study generated the first in vivo active NTMT1 inhibitor for cellular and animal studies. This compound will be a valuable tool for examining the physiological and pharmacological functions of NTMT1 catalytic activity.”
“There is no drug-like inhibitor for NTMT1, a novel but important methyltransferase for cell division and DNA repair,” Huang said. “Our study generated the first in vivo active NTMT1 inhibitor for cellular and animal studies. This compound will be a valuable tool for examining the physiological and pharmacological functions of NTMT1 catalytic activity.”
The first surprise the group discovered was that venglustat, an allosteric inhibitor for glucosylceramide synthase in clinical investigations, can inhibit NTMT1 through a different mechanism. The second surprise was how one hydroxyl group could boost the potency and tune the selectivity for NTMT1 significantly.
Huang added the active inhibitor and negative control will serve as valuable tools to examine the physiological and pharmacological functions of NTMT1 catalytic activity. “Furthermore, GD433 is orally bioavailable and ready to serve as an in vivo probe to elucidate the functions of Nα methylation catalyzed by NTMT1/2 in animal models,” she said.
Next, the Huang Lab continues to work on structural optimization to improve the selectivity further. Meanwhile, they are collaborating on applying NTMT1 inhibitors to investigate the roles of NTMT1 in muscle stem cell differentiation and neurogenesis.
Besides the NTMT1 project, the Huang Lab also focuses on discovering novel inhibitors for protein arginine methyltransferases (PRMTs), Nicotinamide methyltransferase (NNMT), Protein N-terminal acetyltransferases (NATs).

