The Purdue College of Pharmacy honors and recognizes the outstanding research and scholarship generated by our faculty. This publication highlight recognizes Dr. Rodolfo Pinal, for the recent publication, "Tunable Drug Release Rate Using Modular Oral Dosage Forms" which can be read in Pharmaceutics (July, 2023; DOI: 10.3390/pharmaceutics15071905).
Patient-centric medications are around the corner, said a Purdue University Associate Professor of Industrial and Molecular Pharmaceutics.
Rodolfo Pinal recently published a study in Pharmaceutics titled, “Tunable Drug Release Rate Using Modular Oral Dosage Forms,” focused on the opportunities available by applying pharmaceutical manufacturing technologies to fulfill patient-centric pharmacotherapy needs.
 In other words, medications could be customized to release according to a patient’s unique metabolism.
In other words, medications could be customized to release according to a patient’s unique metabolism.
“In the same way food is not metabolized in exactly the same way by different people, drugs in the body are not processed in exactly the same way by every patient,” he said. “One patient can be a slow metabolizer, while another one can be an ultra-fast metabolizer of the same drug. We make oral dosage forms tunable to the needs of individual patients by assembling modules in similar fashion as stacking Lego pieces. Depending on the number, type and order in which modules are stacked, it is possible to readily adjust the dose and speed of release of the drug to match what works best for the individual patient.”
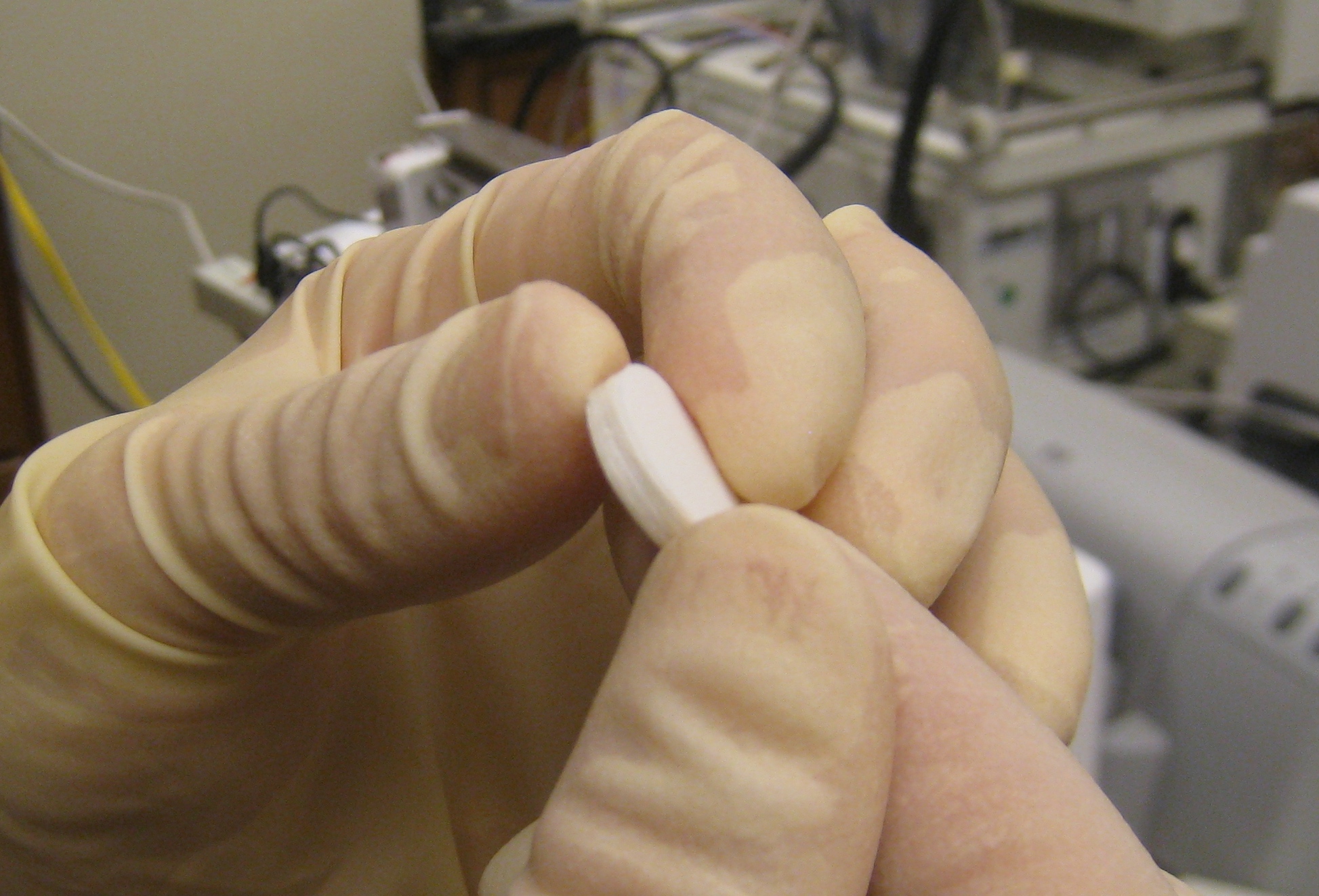
Pinal said the modules used in this study are drug-carrying pharmaceutical films with different speeds of drug release, cut into stackable circular wafers. This makes it possible to tailor the dose and speed-of-release, with high consistency within each specific design.
The current one-dose-fits-all approach to pharmacotherapy dates back to the early 1950s, before the DNA structure was completely clear. Pinal said the prevailing approach is becoming scientifically obsolete for a growing number of therapies. The hangup to moving toward more customized therapies is a gap that exists between biomedical sciences and pharmaceutical manufacturing.
“While the astounding advances in biomedical sciences have taught us much about inter-patient variability in quantitative ways, there is a mismatch with advances in industrial pharmaceutical manufacturing, which has brought improved processes for, and high consistency in, the final one-size-fits-all product,” he said.
The question Pinal seeks to answer is whether it’s possible to bridge that gap in a way that offers better therapy to each patient.
“Every pharmacist and pharmaceutical scientist has an important—even if small—part to play in bringing the best possible therapy to every patient,” he said. “Patient-centric pharmacotherapy is an essential component of the best therapy. Ways to manufacture patient-centric oral dosage forms is one of the many aspects that need to be covered. My research reveals one practicable approach.”
Looking ahead, Pinal said he and his collaborators will move forward in two fronts—deployment and technology.
When it comes to deployment, the modules do not have to be films; modules can be 3D printed layers, increasing efficiency and flexibility. The Purdue College of Pharmacy’s Department of Industrial and Molecular Pharmaceutics has established a collaboration with Aprecia Pharmaceuticals—the only pharmaceutical company in the world with an FDA approved product manufactured by 3D printing. The plan is to pursue a proof-of-concept product for clinical use, likely for pediatric patients.
On the technology front, he said this publication merely presents the simplest possible application of the modular dosage forms approach.
“Additional functionalities, such as solubilizers, absorption enhancers, disintegrants, as well as attributes—such as combination products for the elderly—are aspects for future investigation,” Pinal said.
Pinal’s collaborators on this study were Zhuolin Cong, visiting student from Shenyang Pharmaceutical University in China; Jasmine Liang, PharmD student researcher in the Purdue College of Pharmacy; and Evan Liechty, undergraduate researcher from Purdue’s Department of Chemical Engineering.

